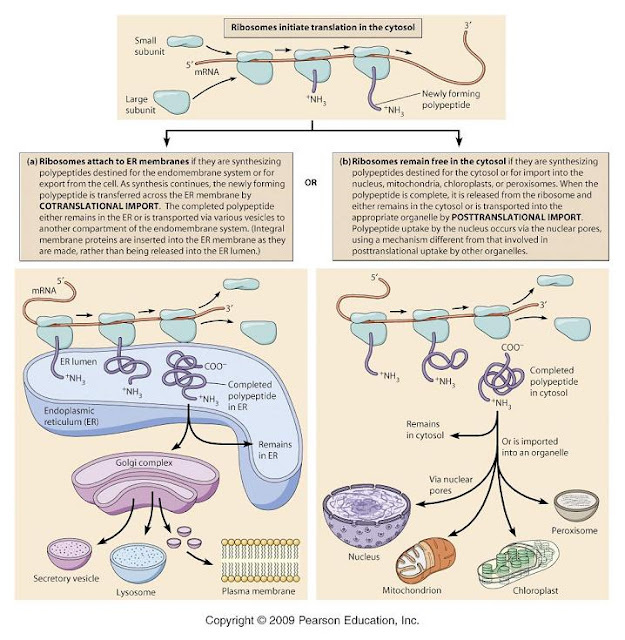
A protein molecule is made up of a long chain of amino acids each linked to its neighbor through a covalent peptide bond. They are therefore referred to as polypeptides.
Amino Acids
Amino acids are the building blocks of proteins. The structure of amino acids consists of a central carbon atom that is bonded to:
- one carboxylic acid group
- one amino group
- a side group (side-chain)
The side group is responsible for the uniqueness of an amino acid since it affects its:
- shape
- size
- composition
- pH
- charge